

| Fall, 1999 excerpt: VYDAC Mobile Phase Modification Guidelines |  |
|---|
The fact that sharp, symmetrical peaks can be obtained on an LC/MS column with lower modifier concentrations does not mean that modifiers have no effect. On the contrary, as shown here for a test mixture of six small peptides, changing modifiers and modifier concentrations can significantly affect not only peak shapes, but retention and selectivity.
The column is Vydac's new 238MS54, a monomerically bonded C18 on 5µm, 300 Å base silica with a proprietary treatment that reduces the concentrations of modifier required. All separations were performed with a 10% to 40% linear gradient of acetonitrile over 30 minutes. As is evident from the chromatograms of Figure 3, significant differences in selectivity arise depending on the concentrations and identities of modifier ions present in the mobile phase, and perfluorinated acidic modifiers even at very low levels (0.005% w/v) can increase retention.
 |
Figure 3. Separation of six small standard
peptides on a Vydac C18 LC/MS column under varying mobile phase conditions.
Sample components: oxytocin, bradykinin, angiotensin II, eledoisin-related peptide, neurotensin, and angiotensin I. Column: Vydac 238MS54 monomeric C18, 5 µm, 300 Å, 4.6mmID x 250mmL. Flow: 1.0 mL/min. Detection: 220 nm. Gradient: Linear, 10% to 40% ACN over 30 minutes for all chromatograms. Mobile phase modifiers as indicated (w/v). |
For this particular mixture, the best spread of peaks was obtained with heptafluorobutyric acid (HFBA) at 0.02% (w/v). However, this might be expected to differ for other peptides.
The new monomeric 238MS54 C18 column produced excellent peak shapes for these peptides and was markedly superior in this regard to the previously reported polymeric 218MS54 C18 LC/MS column (results not shown) with this peptide mixture.
The important lesson: In choosing conditions for HPLC of peptides, a few trial runs with different mobile phases are well worth the effort to determine the best conditions to use for a specific separation.
Vydac LC/MS columns are also available with C4 reversed-phase for proteins
and hydrophobic peptides. Column diameters include 1.0mmID for low-flow
HPLC with direct feed to MS detectors.
| Vydac Cat. # | Description |
|---|---|
| Monomeric C18: (NEW!) | |
| 238MS54 | Column, LC/MS, C18 Reversed Phase, 300Å, 5µm, 4.6mm ID x 250mm L |
| 238MS52 | Column, LC/MS, C18 Reversed Phase, 300Å, 5µm, 2.1mm ID x 250mm L |
| 238MS51 | Column, LC/MS, C18 Reversed Phase, 300Å, 5µm, 1.0mm ID x 250mm L |
| 238MS5.505 | Column, LC/MS, C18 Reversed Phase, 300Å, 5µm, 0.5mm ID x 50mm L |
| 238MS5.510 | Column, LC/MS, C18 Reversed Phase, 300Å, 5µm, 0.5mm ID x 100mm L |
| 238MS5.305 | Column, LC/MS, C18 Reversed Phase, 300Å, 5µm, 0.3mm ID x 50mm L |
| 238MS5.310 | Column, LC/MS, C18 Reversed Phase, 300Å, 5µm, 0.3mm ID x 100mm L |
| Polymeric C18: | |
| 218MS54 | Column, LC/MS, C18 Reversed Phase, 300Å, 5µm, 4.6mm ID x 250mm L |
| 218MS52 | Column, LC/MS, C18 Reversed Phase, 300Å, 5µm, 2.1mm ID x 250mm L |
| 218MS51 | Column, LC/MS, C18 Reversed Phase, 300Å, 5µm, 1.0mm ID x 250mm L |
| 218MS5.505 | Column, LC/MS, C18 Reversed Phase, 300Å, 5µm, 0.5mm ID x 50mm L |
| 218MS5.510 | Column, LC/MS, C18 Reversed Phase, 300Å, 5µm, 0.5mm ID x 100mm L |
| 218MS5.305 | Column, LC/MS, C18 Reversed Phase, 300Å, 5µm, 0.3mm ID x 50mm L |
| 218MS5.310 | Column, LC/MS, C18 Reversed Phase, 300Å, 5µm, 0.3mm ID x 100mm L |
| Polymeric C4: | |
| 214MS54 | Column, LC/MS, C4 Reversed Phase, 300Å, 5µm, 4.6mm ID x 250mm L |
| 214MS52 | Column, LC/MS, C4 Reversed Phase, 300Å, 5µm, 2.1mm ID x 250mm L |
| 214MS51 | Column, LC/MS, C4 Reversed Phase, 300Å, 5µm, 1.0mm ID x 250mm L |
| 214MS5.505 | Column, LC/MS, C4 Reversed Phase, 300Å, 5µm, 0.5mm ID x 50mm L |
| 214MS5.510 | Column, LC/MS, C4 Reversed Phase, 300Å, 5µm, 0.5mm ID x 100mm L |
| 214MS5.305 | Column, LC/MS, C4 Reversed Phase, 300Å, 5µm, 0.3mm ID x 50mm L |
| 214MS5.310 | Column, LC/MS, C4 Reversed Phase, 300Å, 5µm, 0.3mm ID x 100mm L |
Other column sizes are available for analytical and preparative applications.
At the Prep '99 Meeting in San Francisco this past May, researchers from the University of Georgia reported using a recombinant system to produce Aß(1-42) and Aß(1-40) as fusion proteins in E. coli. The fusion proteins are expressed at high levels (average 40 mg/L of culture) and can be rapidly purified by metal-chelating chromatography.
Once the fusion protein has been purified, Aß(1-42) or Aß(1-40) is released by cleavage with Factor Xa protease. The digestion yields the desired protein plus a lesser amount of a truncated protein, Aß(6-42) or Aß(6-40), produced by nonspecific activity of the protease. Some undigested fusion protein may also remain.
A single purification step by reversed-phase chromatography is sufficient for isolation of highly purified product from the cleavage mixture. For Aß(1-40), reversed-phase chromatography was performed under acidic conditions at 60°C on a Vydac low-TFA silica-based C4 or C18 column (Fig. 4).
 |
Figure 4. Separation of digested Aß(1-40)
fusion on silica-based low-TFA reversed phase.
Columns: Vydac 214MS54 C4 and 218MS54 C18. Both 5µm, 300Å, 4.6mmID x 250mmL. Conditions: 60°C, 1.0 mL/min. C4 mobile phase: A = 0.05% TFA in 5% MeCN. B = 0.05% TFA in 95% MeCN. Gradient: Linear 0 to 20% B over 8 minutes. Then 20 to 24% B over 24 minutes. C18 mobile phase: A = 0.075% TFA in 5% MeCN. B = 0.075% TFA in 95% MeCN.Gradient: Linear 0 to 25% B over 8 minutes. Then to 29% B over 24 minutes. Peaks: 1. Aß(1-40); 2. Aß(6-40); 3. uncut fusion. |
For Aß(1-42), purification on silica-based reversed-phase columns was problematic due to low solubility, strong retention, and a tendency to aggregate. These problems could be counteracted by raising the temperature to 80°C in the mildly acid mobile phase, but the high temperature makes the method difficult to scale up for preparative separations. Alternatively, pH above neutrality reduces retention and helps prevent aggregation, but high-pH mobile phases are detrimental to silica-based columns. A Vydac 259VHP54 polymer-based reversed-phase column provided the answer, by virtue of its resistance to high pH. Separation with good yields could be performed at pH 8 and either 60°C or 40°C (Fig. 5). Polymer-based 259VHP columns also have the advantage that they can be cleaned by more aggressive washing solutions.
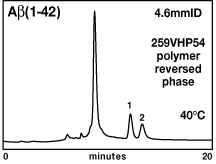 |
Figure 5. Separation of purified digested
Aß(1-42) fusion on polymer-based analytical reversed phase.
Column: Vydac 259VHP54 5µm, 300Å, 4.6mmID x 250mmL. Conditions: 40°C, 1.0 mL/min. Mobile phase: A = 5mM potassium acetate, pH 8.0 in 5% MeCN. B = 5mM potassium acetate, pH 8.0 in 90% MeCN. Gradient: Linear 0 to 20% B over 8 minutes. Then to 26% B over 12 minutes. Peaks: 1. Aß(1-42); 2. Aß(6-42). |
Finally, the reversed-phase separation on 259VHP was scaled up to a 22mmID preparative column. A sample load of 15 mg total protein on that column resulted in a yield of 2 mg of purified Aß(1-42) protein (Fig. 6).
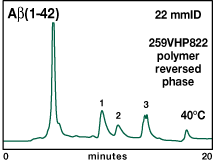 |
Figure 6. Separation of purified digested
Aß(1-42) fusion on polymer-based preparative reversed phase.
Column: Vydac 259VHP822 8µm, 300Å, 22mmID x 250mmL.
Conditions:
40°C, 15 mL/min. Mobile phase: A = 5mM potassium acetate, pH
8.0 in 5% MeCN. B = 5mM potassium acetate, pH 8.0 in 90% MeCN.
Peaks: 1. Aß(1-42); 2. Aß(6-42). |
Purified Aß proteins contain a methionine at position 35 that can become oxidized under certain conditions to yield a sulfoxide form which bears one additional negative charge. The Vydac 259VHP54 column was also able to separate the sulfoxide form from the native form of Aß(1-42), as shown in Figure 7.
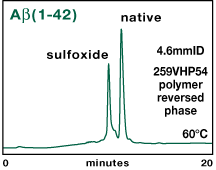 |
Figure 7. Separation of pure Aß(1-42)
peptide, native form and sulfoxide form on polymer-based reversed-phase.
Column: Vydac 259VHP54 5µm, 300Å, 4.6mmID x 250mmL. Conditions: 60°C, 1.0 mL/min. Mobile phase: A = 0.05% TFA in 5% MeCN. B = 0.05% TFA in 90% MeCN. Gradient: Linear 0 to 28% B over 8 minutes. Then to 40% B over 24 minutes. |
*to whom requests for information should be addressed
| Vydac Cat. # | Description |
|---|---|
| 259VHP54 | Column, Polymer Reversed Phase, 300Å, 5µm, 4.6mm ID x 250mm L |
| 259VHP5415 | Column, Polymer Reversed Phase, 300Å, 5µm, 4.6mm ID x 150mm L |
| 259VHP822 | Column, Polymer Reversed Phase, 300Å, 8µm, 22mm ID x 250mm L |
Last Updated: 08/20/21